Tutorial on using the distillation and loss operationsExample - model of ethanol distillation out from fermented mixture using this flowchart editor / process simulator
Before you continue......you should have a basic understanding of the following:Topic and recipe of example projectProduce ethanol from sugar by fermentation Dissolve 30 kg of sugar in 100 litres of aqueous 2.9 g/l sodium citrate buffer solution. Add some fermentation culture to it and let the formed CO2 leave into the air. Then distill off the formed ethanol from the mixture. Tip: try to follow this tutorial with the flowchart editor using example project: Ethanol production by fermentation! Distillation: separate volatile components from less volatile onesDefine distillate composition according to the following:
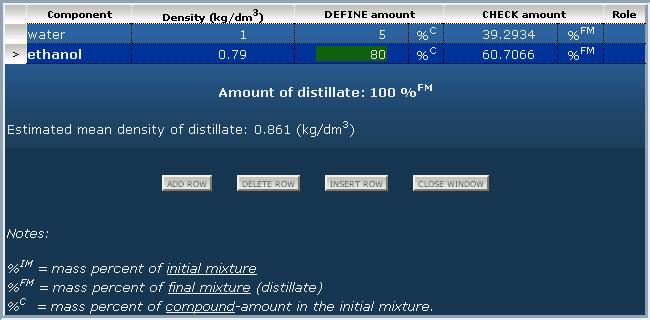
Define composition of the residue by the same method but inversely from the point of view of volatility. The higher amount remains in the residue, the higher amount to set in the composition table. Loss of distillation: uncondensed material as lossSome loss of vapor occurs at every distillation process due to imperfect condensation or/and imperfect sealing. If data is available about this kind of loss, it can be used in this program by the "loss" operation. Before this an output material must be definied for summarizing losses (e.g. "lost compounds into the air" or "exhaust gases into the air"). The composition of lost vapor should be roughly the same as the condensed distillate. In the example below the amount of uncondensed vapor takes about 0.5% of the initial mixture. 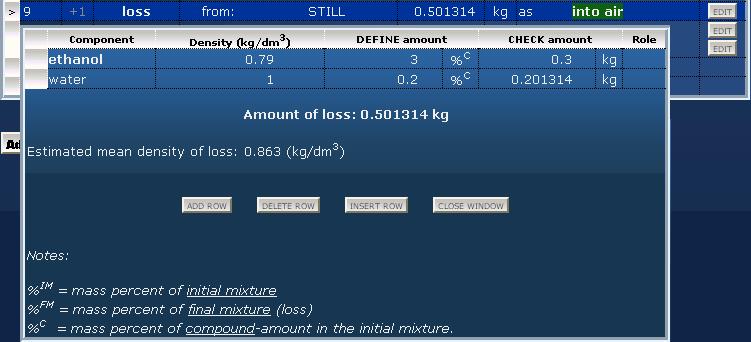
Page top Home |
Useful tools
Engineering ToolBox
The boiling points of some common liquids and gases as acetone, butane, propane, and more
Engineering ToolBox
The boiling points of some common liquids and gases as acetone, butane, propane, and more |
process-flow-diagram.com -- generate chemical technological flowsheets based on material flow calculations
Privacy policy Last update:

